Authors: John C. Douglin, John Varcoe, Dario R. Dekel
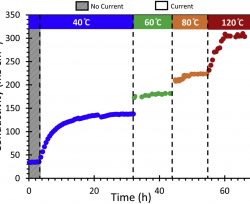
In the past few years, developments in anion exchange membranes (AEMs) have led to a significant increase in hydroxide conductivities, ultimately yielding striking improvements in the performance of anion exchange membrane fuel cells (AEMFCs) at low operating temperatures, usually at 40–80 °C. Aside from these remarkable achievements, the literature is void of any work on AEMFCs operated at temperatures above 100 °C, despite the consensus from various models remarking that working at higher cell temperatures may lead to many significant advantages. In this work, we present the first high-temperature AEMFC (HT-AEMFC) tested at 110 °C. The
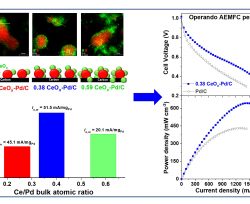
Authors: Ramesh K. Singh, Elena S. Davydova, John Douglin, Andres O. Godoy, Haiyan Tan, Marco Bellini, Bryan J. Allen, Jasna Jankovic, Hamish A. Miller, Ana C. Alba‐Rubio, Dario R. Dekel
Due to the sluggish kinetics of the hydrogen oxidation reaction (HOR) in alkaline electrolytes, the development of more efficient HOR catalysts is essential for the next generation of anion‐exchange membrane fuel cells (AEMFCs). In this work, CeOx is selectively deposited onto carbon‐supported Pd nanoparticles by controlled surface reactions, aiming to enhance the homogenous distribution of CeOx and its preferential attachment to Pd nanoparticles, to achieve highly active CeOx‐Pd/C catalysts. The catalysts are characterized by
Authors: Avital Zhegur-Khais, Fabian Kubannek, Ulrike Krewer, Dario R. Dekel
In this study, a CO2-in-situ purging technique was used to measure the true OH‾ conductivity of several anion exchange membranes (AEMs). During this process, membranes are in-situ de-carbonated allowing the AEMs to reach their full OH‾ form, and therefore to measure their highest (true) OH‾ conductivity. The de-carbonation process in all the studied AEMs was also investigated. The time constant of the de-carbonation process τ was calculated and related to the membrane properties as well as to the de-carbonation dynamics. The time constant of the de-carbonation process was found to decrease with
Authors: Pietro G. Santori, Abhishek N. Mondal, Dario R. Dekel, Frédéric Jaouen
Anion-exchange membrane fuel cells (AEMFCs) show remarkable and rapid progress in performance, significantly increasing the relevance for research on electrocatalysis of the oxygen reduction reaction (ORR) and hydrogen oxidation reaction (HOR) for this technology. Since much of the recent progress in AEMFC performance can be tied to the improved interface between anion-exchange ionomers (AEIs) and catalysts, this topic deserves specific attention. This work reports the ORR and HOR activity measured in rotating disk electrodes for several ionomer–catalyst combinations, involving five different AEIs and Nafion® and four ORR and HOR catalysts selected
Authors: Israel Zadok, Hai Long, Bryan Pivovar, Aleksandra Roznowska, Artur Michalak, Dario R. Dekel, Simcha Srebnik
Understanding the behavior of hydroxide ions in aqueous and non-aqueous media is fundamental to many chemical, biological, and electrochemical processes. Research has primarily focused on a single fully solvated hydroxide ion, either as an isolated cluster or in bulk. This work presents the first computational study to consider hydroxide under low hydration levels in detail, where the anion may not be fully solvated. Under such conditions, we find that the anions are predominantly present as unique water-bridged hydroxide pair complexes, distinct from previously reported structures under
Authors: Nansi Gjineci, Sinai Aharonovich, Sapir Willdorf‐Cohen, Dario R. Dekel, Charles E. Diesendruck
The reaction between different N,N ‐diarylcarbazolium salts and hydroxide is investigated to understand the reaction mechanism. The regioselectivity of the reaction and an unexpected H/D exchange indicate that the reaction does not follow the expected SNAr mechanism, but instead proceeds through a radical pathway.
The mechanism of the reaction between tetraaryl ammonium salts and hydroxide is studied experimentally for different N,N-diaryl carbazolium salts. The N,N-diarylcarbazolium salts are designed, synthesized, characterized, and reacted with hydroxide under different conditions. The products of the reactions were directly characterized or isolated when possible and, using
Authors: Karam Yassin, Igal G. Rasin, Simon Brandon, Dario R. Dekel
During the past decade, one of the main goals of research and development of anion exchange membrane fuel cells (AEMFCs), was to increase the hydroxide conductivity of the anion exchange membranes (AEMs); this goal is based on the obvious and known impact of AEM conductivity on AEMFC performance (including efficiency). We propose a paradigm shift according to which a main AEMFC research goal should be to increase membrane water diffusivity. This is a result of detailed and quantitative computational analyses of AEMFC performance and its stability, presented in this manuscript. Our
Authors: Noam Ralbag, Elena S. Davydova, Meirav Mann-Lahav, Peixi Cong, Jin He, Andrew M. Beale, Gideon S. Grader, David Avnir, Dario R. Dekel
A new heterogeneous catalyst for hydrogen oxidation reaction (HOR), metallic palladium within which nanoparticles of ceria are entrapped, CeO2@Pd, is described. Its preparation is based on a new materials methodology of molecular doping of metals. The metallic matrix, which encages the nanoparticles, is prepared in foam architecture, to ensure easy molecular diffusion. Characterization of the structural properties of the CeO2@Pd composite using SEM, STEM, TEM, XRD, EXAFS and nitrogen adsorption reveals its morphological architecture, which leads to improved catalytic activity. In-situ electrochemical and H2 temperature-programmed reduction
Authors: Avital Zhegur, Nansi Gjineci, Sapir Willdorf-Cohen, Abhishek N. Mondal, Charles E. Diesendruck, Nir Gavish, Dario R. Dekel
Water content plays a major role in the properties of anionexchange membranes (AEMs) and, therefore, in the AEM fuel cell (AEMFC) performance and performance stability. Characterization of AEMs during membrane degradation is critical in order to understand the membrane behavior during fuel cell operation time. In spite of its importance, the relationship between different membrane properties during chemical degradation has yet to be investigated. In this study, we measure the changes in AEM propertiesin particular, ion exchange capacity (IEC), water uptake (WU) and
Authors : Julian Richard Tolchard, Jørgen Svendby, Maidhily Manikandan, Hamish A Miller, Svein Sunde, Hsiharng Yang, Dario R Dekel, Alejandro Oyarce Barnett
The development of active hydrogen oxidation reaction (HOR) and oxygen reduction reaction (ORR) catalysts for use in anion exchange membrane fuel cells (AEMFCs), which are free from platinum group metals (PGMs), is expected to bring this technology one step closer to commercial applications. This paper reports our recent progress developing HOR Pt-free and PGM-free catalysts (Pd/CeO 2 and NiCo/C, respectively), and ORR PGM-free Co 3 O 4 for AEMFCs. The catalysts were prepared by different synthesis techniques and characterized