Synthesis of CeOx-decorated Pd/C catalysts by controlled surface reactions for hydrogen oxidation in anion exchange membrane fuel cells
Authors: Ramesh K. Singh, Elena S. Davydova, John Douglin, Andres O. Godoy, Haiyan Tan, Marco Bellini, Bryan J. Allen, Jasna Jankovic, Hamish A. Miller, Ana C. Alba‐Rubio, Dario R. Dekel
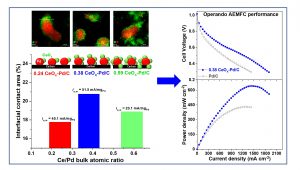
Due to the sluggish kinetics of the hydrogen oxidation reaction (HOR) in alkaline electrolytes, the development of more efficient HOR catalysts is essential for the next generation of anion‐exchange membrane fuel cells (AEMFCs). In this work, CeOx is selectively deposited onto carbon‐supported Pd nanoparticles by controlled surface reactions, aiming to enhance the homogenous distribution of CeOx and its preferential attachment to Pd nanoparticles, to achieve highly active CeOx‐Pd/C catalysts. The catalysts are characterized by inductively coupled plasma–atomic emission spectroscopy, X‐ray diffraction, high‐resolution transmission electron microscopy, scanning transmission electron microscopy (STEM), electron energy loss spectroscopy, and X‐ray photoelectron spectroscopy to confirm the bulk composition, phases present, morphology, elemental mapping, local oxidation, and surface chemical states, respectively. The intimate contact between Pd and CeOx is shown through high‐resolution STEM maps. The oxophilic nature of CeOx and its effect on Pd are probed by CO stripping. The interfacial contact area between CeOx and Pd nanoparticles is calculated for the first time and correlated to the electrochemical performance of the CeOx‐Pd/C catalysts. Highest recorded HOR specific exchange current (51.5 mA mg−1Pd) and H2–O2 AEMFC performance (peak power density of 1,169 mW cm−2 mgPd−1) are obtained with a CeOx‐Pd/C catalyst with Ce0.38/Pd bulk atomic ratio.