Authors: Horie Adabi, Pietro Giovanni Santori, Abolfazl Shakouri, Xiong Peng, Karam Yassin, Igal G. Rasin, Simon Brandon, Dario R. Dekel, Noor Ul Hassan, Moulay-Tahar Sougrati, Andrea Zitolo, John R. Varcoe, John R. Regalbuto, Frederic Jaouen, William E. Mustain
One of the most important needs for the future of low-cost fuel cells is the development of highly active platinum group metal (PGM)-free catalysts. For the oxygen reduction reaction, Fe–N–C materials have been widely studied in both acid and alkaline media. However, reported catalysts in the literature show quite different intrinsic activity and in-cell performance, despite similar synthesis routes and precursors. Here, two types of Fe–N–C are prepared from
Authors: Yogesh Kumar, Elo Kibena-Põldsepp, Jekaterina Kozlova, Mihkel Rähn, Alexey Treshchalov, Arvo Kikas, Vambola Kisand, Jaan Aruväli, Aile Tamm, John C. Douglin, Scott J. Folkman, Ilario Gelmetti, Felipe A. Garcés-Pineda, José Ramón Galán-Mascarós, Dario R. Dekel, and Kaido Tammeveski
Non-precious-metal catalysts are promising alternatives for Pt-based cathode materials in low-temperature fuel cells, which is of great environmental importance. Here, we have investigated the bifunctional electrocatalytic activity toward the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) of mixed metal (FeNi; FeMn; FeCo) phthalocyanine-modified multiwalled carbon nanotubes (MWCNTs) prepared by a simple pyrolysis method. Among the bimetallic catalysts containing nitrogen
Authors: Yabiao Pei, John C. Douglin, Junfeng Zhang, Haoyang Zhao, Jiandang Xue, Qingfa Wang, Ran Li, Yanzhou Qin, Yan Yin, Dario R. Dekel, Michael D. Guiver
Recently, much work has been devoted to designing catalysts with high porosity and efficient active sites. Although very promising results are achieved using Co/Fe–N–C catalysts based on rotating disk electrode (RDE) tests, actual fuel cell performance is below expectations, probably due to insufficient understanding of the catalyst layer (CL). Therefore, catalyst design should be considered holistically by taking into account CL performance, not only intrinsic activity. Here, Co/Fe–N–C with highly dispersed CoFe nanoalloy in the
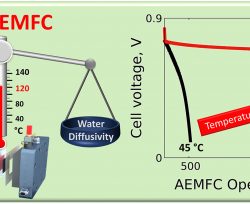
Authors: Karam Yassin, Igal G. Rasin, Sapir Willdorf-Cohen, Charles E. Diesendruck, Simon Brandon, Dario R. Dekel
Anion-exchange membrane fuel cells (AEMFCs) show substantially enhanced (initial) performance and efficiency with the increase of operational temperature (where typical values are below 80 °C). This is directly due to the increase in reaction and mass transfer rates with temperature. Common sense suggests however that the increase of ionomeric material chemical degradation kinetics with temperature is likely to offset the above mentioned gain in performance and efficiency. In this computational study we investigate the combined effect of a high operating temperature, up to 120 °C, on the performance and stability
Authors: Dina Pinsky, Noam Ralbag, Ramesh Kumar Singh, Meirav Mann-Lahav, Gennady E. Shter, Dario R. Dekel, Gideon S. Grader and David Avnir
We developed synthetic methods for the doping of metals (M) with metallic nanoparticles (NPs). To the best of our knowledge – unlike oxides, polymers and carbon-based supports – metals were not used so far as supporting matrices for metallic NPs. The composites (denoted M1-NPs@M2) comprise two separate phases: the metallic NPs (the dopant) and the entrapping 3D porous metallic matrix, within which the NPs are intimately held and well dispersed. Two different general synthetic strategies were developed, each resulting in
Authors: John C. Douglin, Ramesh K. Singh, Saja Haj-Bsoul, Songlin Li, Jasper Biemolt, Ning Yan, John R. Varcoe, Gadi Rothenberg, Dario R. Dekel
We present a first high-temperature anion-exchange membrane fuel cell (HT-AEMFC, operating at 105 °C) based on a critical raw material (CRM)-free cathode catalyst; at the same temperature, the anion-exchange membrane (AEM) has a high ex-situ hydroxide conductivity value of 201 mS cm−1. Our HT-AEMFC, containing a highly active nitrogen-doped carbon (N-doped-C) cathode catalyst, also features low polarization resistances, high catalytic activity and stability, with retention of 81% of the catalyst layer capacitance after an initial 10 h longevity
Authors: Kanika Aggarwal, Saja Bsoul, Songlin Li, Dario R. Dekel, and Charles E. Diesendruck
Long-term stability is a key requirement for anion-exchange membranes (AEMs) for alkaline fuel cells and electrolyzers that is yet to be fulfilled. Different cationic chemistries are being exploited to reach such a goal, and metallopolymers present the unique advantage of chemical stability towards strong nucleophiles as compared to organic cations. Yet, the few metallopolymers tested in strongly alkaline conditions or even in fuel cells still degrade. Therefore, fundamental studies can be advantageous in directing future developments towards this goal. Here, a systematic study of the effect of
Authors: Noam Zion, John C. Douglin, David A. Cullen, Piotr Zelenay, Dario R. Dekel, and Lior Elbaz
Platinum group metal (PGM)-free catalysts for oxygen reduction reaction have shown high oxygen reduction reaction activity in alkaline media. In order to further increase the power density of anion-exchange membrane fuel cells (AEMFCs), PGM-free catalysts need to have a high site density to reach high current densities. Herein, synthesis, characterization, and utilization of heat-treated iron porphyrin aerogels are reported as cathode catalysts in AEMFCs. The heat treatment effect is thoroughly studied and characterized using several techniques, and the best performing aerogel is studied in
Authors: Elena S. Davydova, Maidhily Manikandan, Dario R. Dekel, and Svein Sunde
The latest progress in alkaline anion-exchange membranes has led to the expectation that less costly catalysts than those of the platinum-group metals may be used in anion-exchange membrane fuel cell devices. In this work, we compare structural properties and the catalytic activity for the hydrogen-oxidation reaction (HOR) for carbon-supported nanoparticles of Ni, Ni3Co, Ni3Cu, and Ni3Fe, synthesized by chemical and solvothermal reduction of metal precursors. The catalysts are well dispersed on the carbon support, with particle diameter in the order of 10 nm, and covered by a layer of oxides
Authors: Mamta Kumari, John C. Douglin, Dario R. Dekel
A series of mechanically robust and highly conducting crosslinked anion-exchange membranes are synthesized by using quaternary phosphonium-functionalized poly(ether ether ketone) (QPPEEK) as base polymer and poly(ethylene glycol) (PEG) as the crosslinker. The crosslinked membrane containing both rigid and flexible polymer constituents (QPPEEK-PEG) revealed better flexibility and mechanical strength, and higher conductivity than the pristine QPPEEK membrane. The mechanical properties and ionic conductivity of the crosslinked membranes are tuned by the mass fractions of the QPPEEK and PEG components. Among the different QPPEEK and PEG compositions, the QPPEEK-PEG 20 (QPPEEK:PEG 80:20) membrane has the best