Author: Dario R Dekel
Anion exchange membrane fuel cells (AEMFCs) have recently received increasing attention since in principle they allow for the use of non-precious metal catalysts, which dramatically reduces the cost per kilowatt of power in fuel cell devices. Until not long ago, the main barrier in the development of AEMFCs was the availability of highly conductive anion exchange membranes (AEMs); however, improvements on this front in the past decade show that newly developed AEMs have already reached high levels of conductivity, leading to satisfactory cell performance. In recent years, a growing number of research studies have reported AEMFC performance
Authors : Dario R Dekel, Igal G Rasin, Miles Page, Simon Brandon
We present a new model for anion exchange membrane fuel cells. Validation against experimental polarization curve data is obtained for current densities ranging from zero to above 2 A cm−2. Experimental transient data is also successfully reproduced. The model is very flexible and can be used to explore the system's sensitivity to a wide range of material properties, cell design specifications, and operating parameters. We demonstrate the impact of gas inlet relative humidity (RH), operating current density, ionomer loading and ionomer ion exchange capacity (IEC) values on cell performance. In agreement
Authors : Clémence Lafforgue, Marian Chatenet, Laetitia Dubau, Dario R Dekel
The durability of a state-of-the-art Pt/C electrocatalyst was assessed by accelerated stress test (AST) procedures conducted in liquid alkaline electrolyte (0.1 M NaOH) and in solid anion exchange polymer electrolyte using a “dry cell”, i.e. in absence of liquid electrolyte. In a liquid environment, the positive and negative vertex potential values have a great influence on the extent and on the magnitude of the degradations: the loss of electrochemical surface area observed for a wide potential range (0.1 < E < 1.23 V vs RHE) is ascribed to detachment of
Authors : Travis J Omasta, Xiong Peng, Hamish A Miller, Francesco Vizza, Lianqin Wang, John R Varcoe, Dario R Dekel, William E Mustain
This work reports a high power, stable, completely Pt-free anion exchange membrane fuel cell (AEMFC) comprised of highly active catalysts – Pd-CeO2/C at the anode and PdCu/C alloy at the cathode for the hydrogen oxidation and oxygen reduction reactions, respectively. The resulting AEMFC shows outstanding performance, reaching a peak power density of 1 W cm−2, twice the value of the best performance for Pt-free cells reported in the literature to date. The AEMFC also shows a low voltage degradation rate
Authors : Sapir Willdorf-Cohen, Abhishek N Mondal, Dario R Dekel, Charles E Diesendruck
In recent years, intense research interest has been focused towards the development of anion exchange membrane fuel cells (AEMFCs) due to their potential to circumvent the need for expensive platinum catalysts, tackling the high cost that impedes mass commercialization of fuel cells. However, AEMFCs are not yet practical due to the low chemical stability of the quaternary ammonium (QA) cationic groups during cell operation. Several functionalized polymers for anion exchange membranes (AEMs), including substituted poly(phenylene oxide) (PPO), have been proposed as suitable ionomeric materials, as they present good
Authors : Elena S Davydova, Dario R Dekel
Recent developments in hydroxide conducting anion exchange membranes have contributed to the growing interest in alkaline anion exchange membrane fuel cells (AEMFC) which has the potential to be the next fuel cell generation as in principle this technology allows to replace platinum-based catalysts for affordable metal catalysts. Until now, there are a few research studies describing initial development of hydrogen fueled AEMFC , and practically no work done on completely platinum group metal (PGM)-free catalysts for AEMFC. Though some progress was made in the development of low cost electrocatalysts for oxygen reduction reaction
Authors : Igal G Rasin, Miles Page, Dario R Dekel, Simon Brandon
The implementation of reduced-cost catalyst materials in Anion Exchange Membrane Fuel Cells (AEMFCs) renders them an attractive alternative to Proton Exchange Membrane Fuel Cells (PEMFCs). An important challenge existing in the development of AEMFCs involves water management, which is especially problematic since in these systems (as compared to PEMFCs) water is produced in the anode and consumed in the cathode. We present a model-based analysis of AEMFC performance with an emphasis on water management. Using this approach it is possible to elucidate the relation between reduced performance and issues
Authors: Dario R Dekel, Michal Amar, Sapir Willdorf, Monica Kosa, Shubhendu Dhara, Charles E Diesendruck
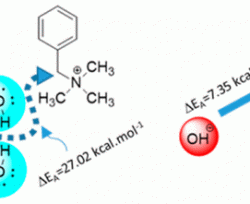
Here we present a novel methodology to measure the alkaline stability of anion conducting polymers to be used as anion exchange membranes and anion exchange ionomers for fuel cells. The new ex situ technique simulates the environment of an anion exchange membrane fuel cell (AEMFC) during operation, where nucleophilic and basic OH– species in the absence, or with a scarce amount of water, attack the functional groups of the ionic polymer. Using this technique, we clearly show the critical effect of water molecules on the alkaline
Authors : Hamish A Miller, Francesco Vizza, Marcello Marelli, Anicet Zadick, Laetitia Dubau, Marian Chatenet, Simon Geiger, Serhiy Cherevko, Huong Doan, Ryan K Pavlicek, Sanjeev Mukerjee, Dario R Dekel
We report an interesting new class of bifunctional electrocatalysts, Pd/C-CeO2, with excellent activity and stability for the hydrogen oxidation reaction (HOR) under alkaline conditions. The unique structure of palladium deposited onto a mixed support of Vulcan XC-72 carbon and CeO2 consists of Pd metal preferable deposited on the ceria regions of the catalyst. The CeO2-Pd interaction leads to enhanced HOR kinetics and increased stability. Here we compare catalysts with three different Pd
Authors : Alina Amel, Nir Gavish, Liang Zhu, Dario R Dekel, Michael A Hickner, Yair Ein-Eli
Quaternary ammonium poly(sulfone) based anion exchange membrane (AEM) in Cl− and HCO3−forms were characterized chemically and morphologically. It was found that the surface of the membrane in both of the forms has highly connective island-like structure, where the diameters of the hydrophilic regions are approximately 5–20 nm. Thermal gravimetric analysis of the membrane in the HCO3− form presented lower decomposition temperatures for the backbone and the side chains, than the membrane in the Cl− form. In addition, the AEM in its HCO3− form showed higher water