Authors: Elena S Davydova, Sanjeev Mukerjee, Frederic Jaouen, Dario R Dekel
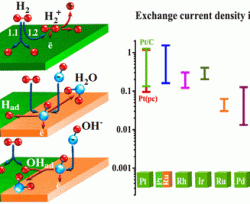
In the past 5 years, advances in anion-conductive membranes have opened the door for the development of advanced anion-exchange membrane fuel cells (AEMFCs) as the next generation of affordable fuel cells. Several recent works have shown that AEMFCs currently achieve nearly identical beginning-of-life performance as state-of-the-art proton exchange membrane fuel cells. However, until now, these high AEMFC performances have been reached with platinum-group metal (PGM)-based anode and cathode catalysts. In order to fulfill the potential of AEMFCs, such catalysts should in the near future be free of PGMs and, eventually,
Authors : Srdjan Pusara, Simcha Srebnik, Dario R Dekel
Much research has focused on the stability of substituted ammonium salts in anion-exchange membranes (AEMs). While cation chemistry dictates AEM stability, chemical degradation has been recently shown to be significantly influenced by the hydration level at which the AEM operates. At low hydration, it is now known that almost every quaternary ammonium may suffer significant decomposition. In this work, we use molecular dynamics simulations to explore the behavior of three common quaternary ammonium cations with stoichiometric hydroxide concentration and at very low hydration. We find that water preferentially solvates hydroxide anions and
Authors: Yiwei Zheng, Uri Ash, Ravi P Pandey, Amobi G Ozioko, Julia Ponce-González, Michael Handl, Thomas Weissbach, John R Varcoe, Steven Holdcroft, Matthew W Liberatore, Renate Hiesgen, Dario R Dekel
Anion exchange membrane fuel cells (AEMFCs) have attracted extensive attention in the recent years, primarily due to the distinct advantage potentials they have over the mainstream proton exchange membrane fuel cells. The anion exchange membrane (AEM) is the key component of AEMFC systems. Because of the unique characteristics of water management in AEMFCs, understanding the water mobility through AEMs is key for this technology, as it significantly affects (and limits) overall
Authors : Clémence Lafforgue, Marian Chatenet, Robert W Atkinson, Karen Swider-Lyons, Hamish Miller,
Dario R Dekel
Direct borohydride fuel cells (DBFC) are considered high-energy density generators. The ideal case for the anodic reaction of a DBFC is the direct and complete borohydride oxidation reaction (BOR) that releases 8 electrons. However, the BOR is a very complex reaction that involves numerous intermediate species and suffers from competition with the heterogeneous hydrolysis of BH4- that leads to molecular hydrogen, making full utilization of the fuel challenging . Despite many studies of the BOR on several electrocatalysts (especially Au and Pt), this reaction is still
Authors : Clémence Lafforgue, Laetitia Dubau, Frederic Maillard, Dario R Dekel, Marian Chatenet
Alkaline fuel cells (AFC) are competitors to proton-exchange membrane fuel cells for stationary applications . Because many metals and metal oxides are stable at high pH , one may think that AFC electrocatalysts will be more stable in operation than in acidic medium. However, this was proven wrong for carbon-supported Pt and Pd nanoparticles (NPs) aged in liquid alkaline environment: these undergo severe electrochemical surface area losses even for a very mild potential cycling procedure in 0.1 M NaOH . Identical-location transmission electron microscopy (ILTEM) experiments revealed pronounced
Authors : Noga Ziv, William E Mustain, Dario R Dekel
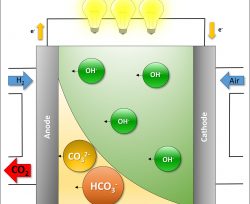
Over the past 10 years, there has been a surge of interest in anion‐exchange membrane fuel cells (AEMFCs) as a potentially lower cost alternative to proton‐exchange membrane fuel cells (PEMFCs). Recent work has shown that AEMFCs achieve nearly identical performance to that of state‐of‐the‐art PEMFCs; however, much of that data has been collected while feeding CO2‐free air or pure oxygen to the cathode. Usually, removing CO2 from the oxidant is done to avoid the detrimental effect of CO2 on AEMFC performance, through carbonation, whereby CO2 reacts with the OH− anions to form
Authors: Noga Ziv, Dario R Dekel
Hydroxide ions in anion exchange membranes (AEMs) are quickly exchanged for larger and less mobile anions (CO₃2− and HCO₃−) when the membrane is exposed to ambient air. Therefore, reported conductivity values of AEMs in hydroxide form are difficult to reproduce, and existing conductivity measurement techniques are not always reliable. Up to now, comparison of reported data for the hydroxide conductivity of different membranes has not been possible because tests have been performed not just with different anions, but also under different conditions and using different methods. In this work we present a practical and reproducible
Authors : Ulrike Krewer, Christine Weinzierl, Noga Ziv, Dario R Dekel
Alkaline anion exchange membrane fuel cell (AEMFC) is a promising technology to replace precious metals used today as fuel cell catalysts. However, AEMFC does not yet demonstrate high performance when running on ambient air where they are exposed to CO2. The resulting carbonation reaction reduces membrane conductivity. This paper analyses and quantifies the effect of CO2 from ambient air on the concentration profiles in the membrane and the anode and, thus, assesses the CO2 impact on fuel cell performance. The physico-chemical model contains chemical and electrochemical reactions, liquid-gas phase equilibria
Authors : Shimshon Gottesfeld, Dario R Dekel, Miles Page, Chulsung Bae, Yushan Yan, Piotr Zelenay, YS Kim
The anion exchange membrane fuel cell (AEMFC) is an attractive alternative to acidic proton exchange membrane fuel cells, which to date have required platinum-based catalysts, as well as acid-tolerant stack hardware. The AEMFC could use non-platinum-group metal catalysts and less expensive metal hardware thanks to the high pH of the electrolyte. Over the last decade, substantial progress has been made in improving the performance and durability of the AEMFC through the development of new materials and the optimization of system design and operation conditions.
Authors : Dario R Dekel, Sapir Willdorf, Uri Ash, Michal Amar, Srdjan Pusara, Shubhendu Dhara, Simcha Srebnik, Charles E Diesendruck
Anion exchange membrane fuel cells can potentially revolutionize energy storage and delivery; however, their commercial development is hampered by a significant technological impedance: the chemical decomposition of the anion exchange membranes during operation. The hydroxide anions, while transported from the cathode to the anode, attack the positively charged functional groups in the polymer membrane, neutralizing it and suppressing its anion-conducting capability. In recent years, several new quaternary ammonium salts have been proposed to address this challenge, but while they perform well