Authors: Jasmin Muller, Avital Zhegur, Ulrike Krewer, John R Varcoe, Dario R Dekel
Anion-exchange membrane (AEM) degradation during fuel cell operation represents the main challenge that hampers the implementation of AEM fuel cells (AEMFCs). Reported degradation values of AEMs are difficult to reproduce as no standard methods are used. The present use of different techniques based on exposure of membranes to aqueous KOH solutions under different conditions and measuring different outputs during time does not allow for a reliable and meaningful comparison of reported degradation data of different AEMs. In this study, we present a practical and reproducible ex-situ technique to
Authors : Tong Huang, Guangwei He, Jiandang Xue, Obed Otoo, Xueyi He, Haifei Jiang, Junfeng Zhang, Yan Yin, Zhongyi Jiang, John C Douglin, Dario R Dekel, Michael D Guiver
Combining two facile methods, spinodal blending and self-crosslinking by chloromethyl groups are jointly utilized in fabricating alkaline anion exchange membranes (AEMs). Highly and moderately chloromethylated poly(ether ether ketone)s and sub-equimolar (to chloromethyl) amounts of 1-methylimidazole are mixed and reacted to form blend AEMs. Nanoscale bi-continuous phase separated morphologies are obtained due to spinodal decomposition. Subsequent heat treatment triggers self-crosslinking of the AEMs arising from residual chloromethyl groups in membranes. Compared with unblended
Effect of Ammonium Cations on the Diffusivity and Structure of Hydroxide Ions in Low Hydration Media
Authors : Israel Zadok, Dario R Dekel, Simcha Srebnik
Anion exchange membrane (AEM) fuel cells are an attractive alternative technology to the acidic proton exchange membrane-based fuel cells. Conduction of hydroxide ions in AEMs creates an alkaline operating environment that allows using platinum-free catalysts, while still maintaining the performance needed for commercial application (e.g., the automotive industry). However, this technology is very sensitive to the behavior of hydroxide ions under low hydration conditions because of the consumption of water near the cathode. We use molecular dynamics simulation to investigate the behavior of two model quaternary ammonium cations used in AEM technologies
Authors: Noam Ralbag, Meirav Mann-Lahav, Elena S Davydova, Uri Ash, Reuven Galed, Michael Handl, Renate Hiesgen, Emanuele Magliocca, William Mustain, Jin He, Peixi Cong, Andrew M Beale, Gideon S Grader, David Avnir, Dario R Dekel
In this work, we develop a new type of composite material that combines both electrocatalytic and ionic properties, by doping a silver metal catalyst with an anion-conducting ionomer at the molecular level. We show that ionomer entrapment into the silver metallic structure is possible, imparting unique properties to the catalytic character of the metallic silver. The novel composite material is tested as the cathode electrode of
Authors : Tamar Zelovich, Leslie Vogt-Maranto, Michael A Hickner, Stephen J Paddison, Chulsung Bae,
Dario R Dekel, Mark E Tuckerman
Operation of anion-exchange membrane (AEM) fuel cells (AEMFCs) results in gradients in the cell that can lead to low-hydration conditions within the cell. It is therefore important to investigate hydroxide ion diffusion in AEMs with low water-to-cation ratios (λ ≤ 4, λ≡nH2O/ncation). In this work, ab initio molecular dynamics simulations are presented to explore hydroxide ion solvation complexes and diffusion mechanisms in model AEMs at low hydration. By changing the cation spacing within the AEM and the degree of hydration, six different
Authors: Marco Bellini, Maria Vincenza Pagliaro, Anna Lenarda, Paolo Fornasiero, Marcello Marelli, Claudio Evangelisti, Massimo Innocenti, Qingying Jia, Sanjeev Mukerjee, Jasna Jankovic, Lianqin Wang, John Robert Varcoe, Chethana Bhadravathi Krishnamurthy, Ilya Grinberg, Elena Davydova, Dario R Dekel, Hamish Andrew Miller, Francesco Vizza
Anion exchange membrane fuel cells (AEMFCs) offer several important advantages with respect to proton exchange membrane fuel cells, including the possibility of avoiding the use of platinum catalysts to help overcome the high cost of fuel cell systems. Despite such potential benefits, the slow kinetics of the hydrogen oxidation reaction (HOR) in alkaline media and limitations in performance stability (because
Authors: Simcha Srebnik, Srdjan Pusara, Dario R Dekel
Currently, there are two main challenges in state-of-the-art anion-exchange membrane fuel cells (AEMFCs)—first, cation degradation in the presence of hydroxide anions; second, carbonation process during AEMFC operation. Both degradation and carbonation processes lead to a significant decrease in the ionic conductivity of the anion exchange membranes (AEMs), and, in turn, in the AEMFC performance. In this work, we use molecular dynamics simulations to bring first insights into the contributing factors that lead to changes in the degradation of quaternary ammonium cations due to the presence of carbonate anions. Focusing on low hydration levels,
Authors: Elena Davydova, Florian D Speck, Michael TY Paul, Dario R Dekel, Serhiy Cherevko
Among the non-noble-metal electrocatalysts for the hydrogen oxidation reaction (HOR) in anion exchange membrane fuel cells (AEMFCs), Ni-based nanoparticles have shown the highest reported activities. In this work, we investigated the chemical and electrochemical stability of representative Ni-based electrocatalysts. For this, carbon-supported monometallic Ni and bimetallic Ni3M (M = Co, Fe, Cu, Mo) nanoparticles were synthesized and tested using a set of complementary techniques. It was found that Mo suffers from intense dissolution due to thermodynamic instability. Cu was stable below 0.4 VRHE, though it undergoes noticeable
Authors :Jiantao Fan, Sapir Willdorf-Cohen, Eric M Schibli, Zoe Paula, Wei Li, Thomas JG Skalski, Ania Tersakian Sergeenko, Amelia Hohenadel, Barbara J Frisken, Emanuele Magliocca, William E Mustain, Charles E Diesendruck, Dario R Dekel, Steven Holdcroft
Solid polymer electrolyte electrochemical energy conversion devices that operate under highly alkaline conditions afford faster reaction kinetics and the deployment of inexpensive electrocatalysts compared with their acidic counterparts. The hydroxide anion exchange polymer is a key component of any solid polymer electrolyte device that operates under alkaline conditions. However, durable hydroxide-conducting polymer electrolytes in highly caustic media have proved elusive, because polymers bearing cations are
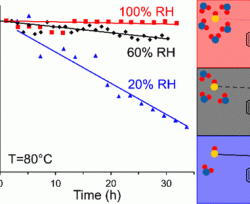
Authors : Noga Ziv, Abhishek N Mondal, Thomas Weissbach, Steven Holdcroft, Dario R Dekel
In this study the effect of CO2, HCO3‾ and CO322‾ on the ionic conductivity and water uptake properties of anion exchange membranes (AEMs) was investigated in order to better understand the detrimental effect of ambient air feed on the performance of AEM fuel cells. Three types of AEMs were examined, including Poly(hexamethyl-pp-terphenyl benzimidazolium) (HMT-PMBI), Fumatech® FAA-3, and poly(phenylene oxide) functionalized with imidazole (PPO-Im). The effect of temperature and humidity on AEM properties in their different anion forms was studied, including both steady state and dynamic measurements. In