Here we present a novel methodology to measure the alkaline stability of anion conducting polymers to be used as anion exchange membranes and anion exchange ionomers for fuel cells. The new ex situ technique simulates the environment of an anion exchange membrane fuel cell (AEMFC) during operation, where nucleophilic and basic OH– species in the absence, or with a scarce amount of water, attack the functional groups of the ionic polymer. Using this technique, we clearly show the critical effect of water molecules on the alkaline stability of quaternary ammonium (QA) cations commonly used as functional groups in AEMFCs. The
Articles
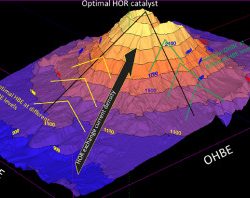
Anion exchange membrane fuel cells (AEMFCs) can potentially revolutionize the energy generation market; however, to be seriously considered as a real alternative to the mainstream fuel cell technology, complete removal of previous metal electrocatalysts needs to be achieved. While in cathode electrodes platinum can be easily substituted, the electrochemical hydrogen oxidation reaction (HOR) in the AEMFC anodes currently involves prohibitive overpotential losses, making the removal of platinum extremely challenging. Understanding the HOR in AEMFCs will facilitate the path to overcome the challenge and finally develop and demonstrate platinum-free high-performance AEMFC devices.
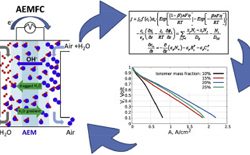
We present a new model for anion exchange membrane fuel cells. Validation against experimental polarization curve data is obtained for current densities ranging from zero to above 2 A cm−2. Experimental transient data is also successfully reproduced. The model is very flexible and can be used to explore the system's sensitivity to a wide range of material properties, cell design specifications, and operating parameters. We demonstrate the impact of gas inlet relative humidity (RH), operating current density, ionomer loading and ionomer ion exchange capacity (IEC) values on cell performance. In agreement with the literature, high air RH levels are shown to improve
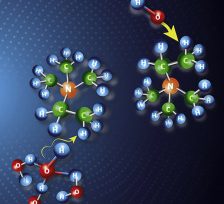
Anion exchange membrane fuel cells can potentially revolutionize energy storage and delivery; however, their commercial development is hampered by a significant technological impedance: the chemical decomposition of the anion exchange membranes during operation. The hydroxide anions, while transported from the cathode to the anode, attack the positively charged functional groups in the polymer membrane, neutralizing it and suppressing its anion-conducting capability. In recent years, several new quaternary ammonium salts have been proposed to address this challenge, but while they perform well in ex-situ chemical studies, their performance is very limited in real fuel cell studies. Here, we use experimental work,
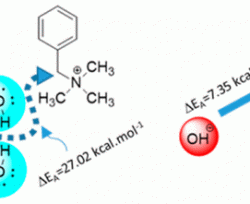
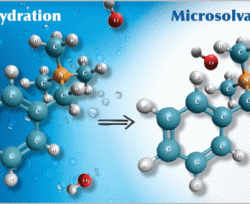
Much research has focused on the stability of substituted ammonium salts in anion-exchange membranes (AEMs). While cation chemistry dictates AEM stability, chemical degradation has been recently shown to be significantly influenced by the hydration level at which the AEM operates. At low hydration, it is now known that almost every quaternary ammonium may suffer significant decomposition. In this work, we use molecular dynamics simulations to explore the behavior of three common quaternary ammonium cations with stoichiometric hydroxide concentration and at very low hydration. We find that water preferentially solvates hydroxide anions and hence when water is present in sufficient amount
Anion exchange membrane fuel cells (AEMFCs) have recently received increasing attention since in principle they allow for the use of non-precious metal catalysts, which dramatically reduces the cost per kilowatt of power in fuel cell devices. Until not long ago, the main barrier in the development of AEMFCs was the availability of highly conductive anion exchange membranes (AEMs); however, improvements on this front in the past decade show that newly developed AEMs have already reached high levels of conductivity, leading to satisfactory cell performance. In recent years, a growing number of research studies have reported AEMFC performance results. In the